FIRST AND ONLY READY-TO-USE
VIGABATRIN ORAL SOLUTION
Ready-to-use. No mixing required and is designed to:
- Provide a consistently correct concentration
- Help eliminate mixing errors
- Limit risk of contamination*



Note: Not actual size. Syringe and bottle adapter provided by pharmacy.
Helping to eliminate errors in dosing preparation
VIGAFYDE is indicated as monotherapy for pediatric patients with infantile spasms 1 month to 2 years of age for whom the potential benefits outweigh the potential risk of vision loss.
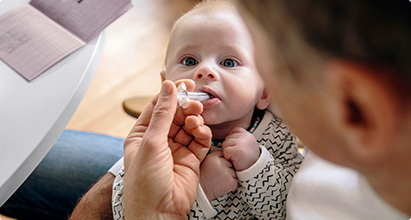
For patients with infantile spasms, VIGAFYDE provides a consistently correct concentration (100 mg/mL vigabatrin oral solution).
No reconstitution (mixing) by the caregiver is needed, which means reduced dose preparation time, no medication waste, and may help reduce the risk of preparation errors.
Note: Not actual size. Syringe and bottle adapter provided by pharmacy.
VIGAFYDE is indicated as monotherapy for pediatric patients with infantile spasms 1 month to 2 years of age for whom the potential benefits outweigh the potential risk of vision loss.
For patients with infantile spasms, VIGAFYDE provides a consistently correct concentration (100 mg/mL vigabatrin oral solution).
No reconstitution (mixing) by the caregiver is needed, which means reduced dose preparation time, no medication waste, and may help reduce the risk of preparation errors.
See the VIGAFYDE difference
*After one-time insertion of the adaptor, supplies include an oral syringe and a 150 mL bottle of ready-to-use oral solution.
† Vigabatrin powder for oral solution. Supplies include the number of product packets needed for each dose; 2 clean cups, 1 for mixing and 1 for water (the cup used for mixing vigabatrin should be clear in order to see if the powder is dissolved); water to mix the vigabatrin powder; one small 3 mL oral syringe and one large 10 mL oral syringe, which are provided by the pharmacy; a small spoon or other clean utensil to stir the mixture; and scissors to cut the powder packet. The number of product packets needed for each dose will vary by prescribed dosage.

Starting patients on VIGAFYDE
Healthcare providers must be certified in the Vigabatrin REMS Program in order to prescribe VIGAFYDE.
The US Food and Drug Administration (FDA) has approved a single shared system Risk Evaluation and Mitigation Strategy (REMS) for all vigabatrin products, called the Vigabatrin REMS Program.
The Vigabatrin REMS Program is required by the FDA to ensure informed risk-benefit decisions before initiating treatment, and to ensure appropriate use of vigabatrin while patients are treated. If you are already certified for vigabatrin products, you are ready to prescribe VIGAFYDE.
VIGAFYDE can be e-prescribed through your EMR, or you can complete the Prescription and Enrollment form to start the process.